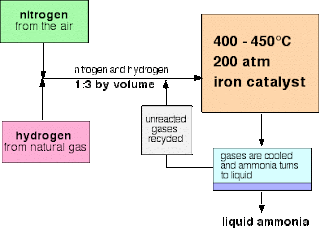
i
a temperature of about 450°C
ii a pressure of about 200 atmospheres
iii an iron catalyst
Key equation:
N2(g)
+ 3H2(g)
⇌ 2NH3(g)
More
facts to learn!
Why does
the Haber Process operate at 200
atm and 450 ºC?
High pressure is good. It increases the yield (as explained above), and also the rate. We don’t
make the pressure higher still, because the increased cost of doing that is not worth the relatively
low extra increase
in yield and rate.
High temperature is bad for the yield (as explained
above). However, we use a high temperature to get a
reasonable rate. It is a compromise between yield and rate. There is
no point in having a yield of
100 % if it takes all month
to make one tonne of ammonia! (This is why the catalyst is so useful. If we didn’t use a catalyst, the temperature would have to be higher still
to get a reasonable rate, and the yield
would be even lower).
No comments:
Post a Comment