Simple distillation
This is used
to separate a liquid from dissolved solids
when we wish to collect
the liquid, e.g., pure water
from sea water.
Distillation involves boiling the liquid so it
turns to vapour and then condensing the vapour back to liquid, which is
collected as the distillate. The
dissolved solids remain in the flask.
Fractional distillation
 The addition of a fractionating column helps separate
liquids with different boiling
points, e.g., a mixture of ethanol and water.
The addition of a fractionating column helps separate
liquids with different boiling
points, e.g., a mixture of ethanol and water.
The liquid with the lower boiling point
evaporates more easily and rises through the fractionating column first. The thermometer at the top shows the boiling
point of the component that is coming through to be condensed in the
condenser. The distillate, known as a
fraction, is collected in the flask.
The liquid with the higher boiling point will
condense in the fractionating column and drip back into the flask at
first. This will come through later as
the temperature rises.
Filtration
An example is separating unreacted copper (II)
oxide from a solution of copper (II) sulfate after reaction with sulfuric acid
(see section on preparing salts: 4.7)
An evaporating basin is used to separate a soluble
solid from a liquid when we do not wish to collect the liquid. The last part of the evaporation must be carried out slowly to form decent crystals.
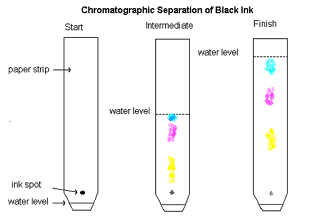
Chromatography
Used for analysis.
It separates a mixture of coloured
dyes or inks which show up as dots on chromatography paper at
different heights.
The solvent, e.g. water, must be below the sample spot as shown
opposite.



No comments:
Post a Comment